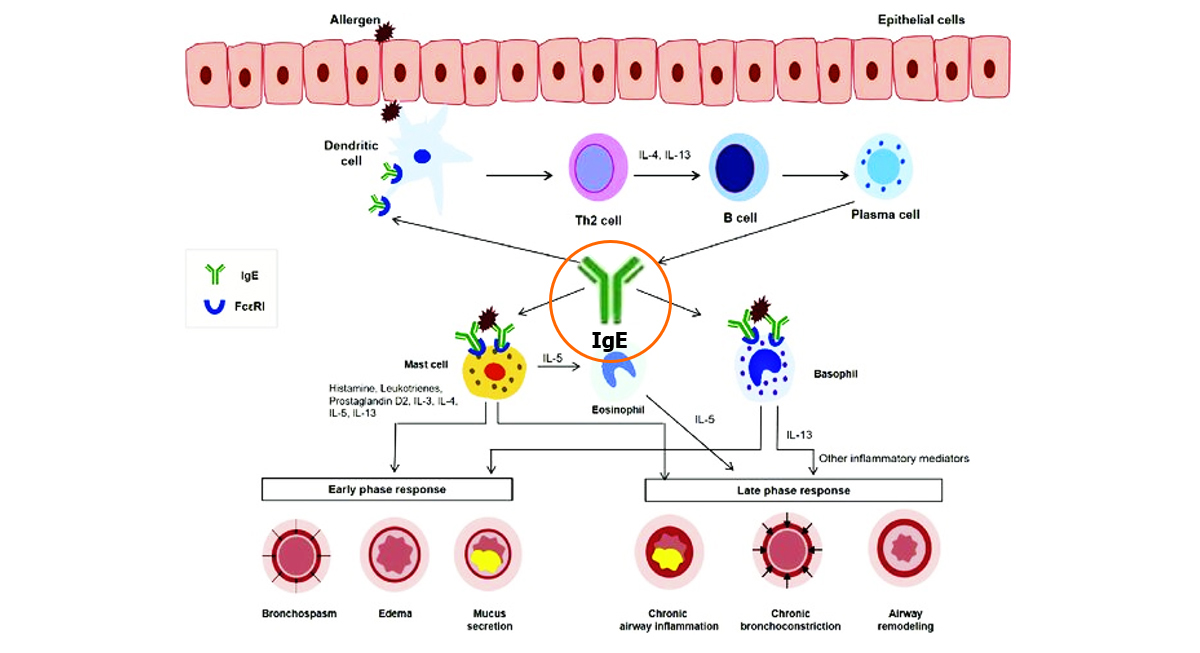
Immunological mechanisms in IgE-mediated allergic diseases
Source: Humbert M , Bousquet J , Bachert C ,et al.IgE-Mediated Multimorbidities in Allergic Asthma and the Potential for Omalizumab Therapy[J].
The Journal of Allergy and Clinical Immunology In Practice, 2019, 7(5).DOI:10.1016/j.jaip.2019.02.030
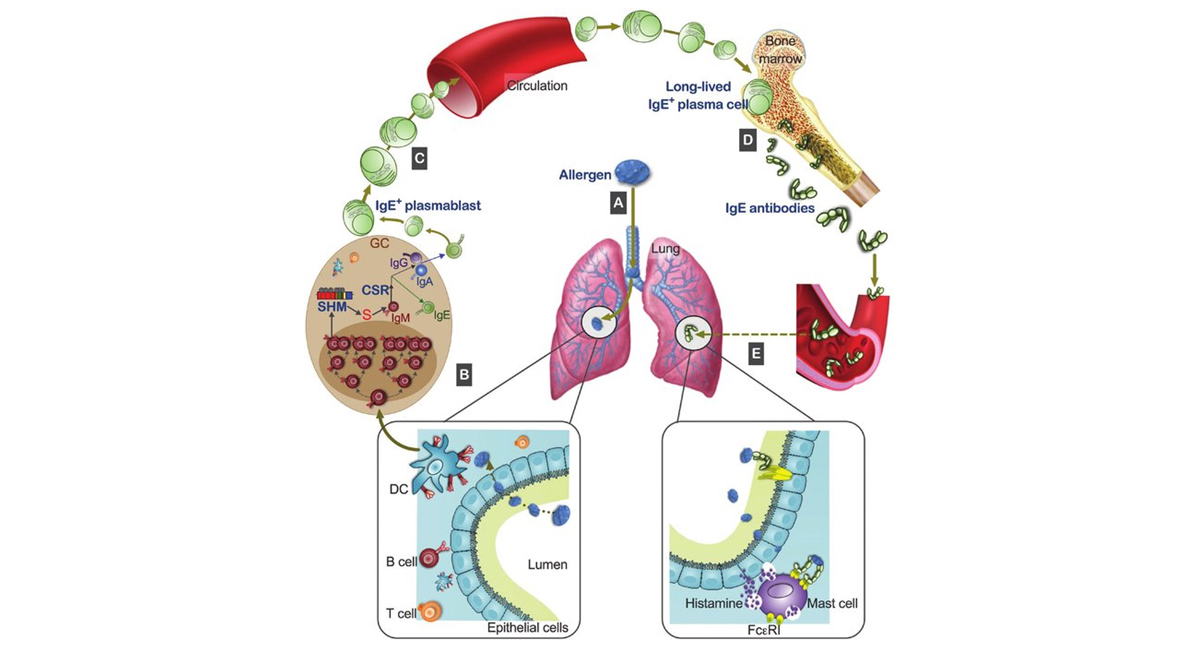
Compartmental regulation and IgE function in allergic asthma
Source: Gould H J , Bryan Y C .IgE repertoire and immunological memory:
Compartmental regulation and antibody function[J].International Immunology,2018(9):9.DOI:10.1093/intimm/dxy048.

*KinExA assay
【A】Gaser, ,Tarchevskaya,s.s,Guntemn,P, etal. The mechanistic and functional profile of thetherapeutic antllg antibody ligelizumab difers from omalzumab. NatCommun 11,165 (2020).https://doi.org/10.1038/s41467-019-13815-W
【B】Xolair (omalizumab for subcutaneous use)label. Available at https:/www.accessdata.fda.gov/drugsatfda_docs/label/2023/103976s5242lbl.pdf 23 Jan 2024,date last accessed).”
【C】Arm JP, Bottolil, skerianecA ,et al.Phamacokinetics, pharmacodynamics and safety of0GE031 ligelizumab, a novel high - afinity anti - lE antibody,in atopisubjects[J].Clinical & Experimental Allergy, 2014, 44(11).D01:10.1111/cea.12400.